We combine computational and circuit-level neuroscience to understand reinforcement learning and plasticity, identifying novel prediction error mechanisms that disrupt maladaptive behavior in psychiatric diseases.
ongoing projects
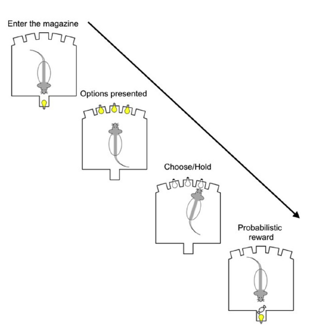
Decision-Making and Reinforcement Learning: We investigate the limbic cortico-striatal circuitry that underlies flexible, habitual, and model-based/model-free reinforcement learning, as well as inhibitory control. We employ computational modeling and machine learning to dissect distinct mechanisms that result in individual differences in decision-making and relate these to vulnerability to addiction and psychosis.
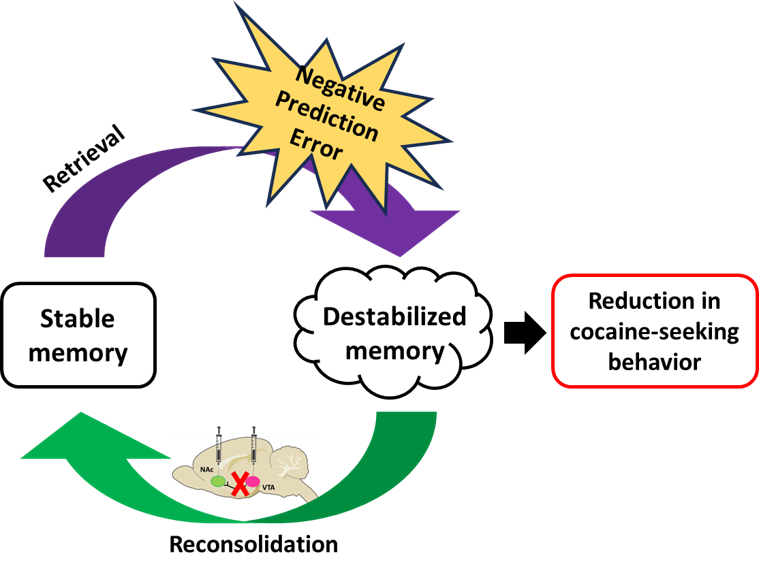
Addiction and Memory Plasticity: We focus on the persistent neuroadaptations associated with substance use disorders and alcoholism. A key area involves manipulating prediction errors to enhance memory destabilization and reconsolidation, with the aim of reducing drug-seeking behavior and destabilizing cue-drug memories. We are interested in memory plasticity processes (destabilization and restabilization) that are involved in memory reconsolidation, which allows new information to be integrated into memory and cognition.
Novel Therapeutics: We explore the effects of compounds like psychedelics and entactogens on animal models of addictions, depression, learning, motivation, and decision-making processes. Together, we attempt to understand neurotrophin/glutamatergic/dopaminergic signaling molecules/alterations in limbic cortico-striatal function relevant to stress, addictions/alcohol, learning and memory processes and vulnerability to psychiatric dysfunction.
investigating prosocial decision-making
social neurocircuitry and psychiatry
Led by Will Li and Henry Kietzman, a dedicated theme of our research focuses on the neural circuits driving social decision-making, particularly cooperation, competition, and the factors that bias an agent towards self-directed and prosocial strategies.
Cooperation Assay: We utilize a novel, semi-naturalistic rodent cooperation assay (a paired decision-making task) to model the appraisal and coordination of actions required for mutual reward. This allows us to dissect the neural basis of social cooperation and the lasting structural and functional changes it induces.
Circuit Plasticity and Dynamics: We investigate how repeated social cooperation drives plasticity, finding lasting structural change (e.g., increased synaptic density in the insula and amygdala) and revealing opposing roles in corticolimbic populations.
Early Life Adversity and Intervention: A major focus is on the impact of ELA on socio-behavioral dysfunction. We use our novel paired assay to identify ELA-specific deficits and to probe whether behavioral and pharmacological interventions, including entactogens, are sufficient to restore cooperative function, aiming to ameliorate downstream socio-behavioral challenges.
selected publications
-
Li, S. W., Kietzman, H. W., Taylor, J. R., & Chang, S. W. C. (2025). The Evolving Landscape of Social Neuroscience and Its Implications for Psychiatry. Biological psychiatry, 97(10), 936–938. https://doi.org/10.1016/j.biopsych.2024.06.004
-
Trinko, J. R., Foscue, E., Kong, E. M., Basu, A., Corstens, A. M., Thompson, S. L., Kaye, A. P., Taylor, J. R., & DiLeone, R. J. (2024). Xylazine induces dopamine release and augments the effects of fentanyl. The Journal of clinical investigation, 134(22), e183354. https://doi.org/10.1172/JCI183354
Suthaharan, P., Thompson, S. L., Rossi-Goldthorpe, R. A., Rudebeck, P. H., Walton, M. E., Chakraborty, S., Noonan, M. P., Costa, V. D., Murray, E. A., Mathys, C. D., Groman, S. M., Mitchell, A. S., Taylor, J. R., Corlett, P. R., & Chang, S. W. C. (2024). Lesions to the mediodorsal thalamus, but not orbitofrontal cortex, enhance volatility beliefs linked to paranoia. Cell reports, 43(6), 114355. https://doi.org/10.1016/j.celrep.2024.114355
-
Asch, R. H., Fowles, K., Pietrzak, R. H., Taylor, J. R., & Esterlis, I. (2023). Examining mGlu5 Receptor Availability as a Predictor of Vulnerability to PTSD: An [18F]FPEB and PET Study in Male and Female Rats. Chronic stress (Thousand Oaks, Calif.), 7, 24705470231215001. https://doi.org/10.1177/24705470231215001
Asch, R. H., Fowles, K., Pietrzak, R. H., Taylor, J. R., & Esterlis, I. (2023). Examining mGlu5 Receptor Availability as a Predictor of Vulnerability to PTSD: An [18F]FPEB and PET Study in Male and Female Rats. Chronic stress (Thousand Oaks, Calif.), 7, 24705470231215001. https://doi.org/10.1177/24705470231215001
Moin Afshar, N., Cinotti, F., Martin, D., Khamassi, M., Calu, D. J., Taylor, J. R., & Groman, S. M. (2023). Reward-Mediated, Model-Free Reinforcement-Learning Mechanisms in Pavlovian and Instrumental Tasks Are Related. The Journal of neuroscience : the official journal of the Society for Neuroscience, 43(3), 458–471. https://doi.org/10.1523/JNEUROSCI.1113-22.2022
Asch, R. H., Pothula, S., Toyonaga, T., Fowles, K., Groman, S. M., Garcia-Milian, R., DiLeone, R. J., Taylor, J. R., & Esterlis, I. (2023). Examining sex differences in responses to footshock stress and the role of the metabotropic glutamate receptor 5: an [18F]FPEB and positron emission tomography study in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 48(3), 489–497. https://doi.org/10.1038/s41386-022-01441-y
-
Lee, B., Pothula, S., Wu, M., Kang, H., Girgenti, M. J., Picciotto, M. R., DiLeone, R. J., Taylor, J. R., & Duman, R. S. (2022). Positive modulation of N-methyl-D-aspartate receptors in the mPFC reduces the spontaneous recovery of fear. Molecular psychiatry, 27(5), 2580–2589. https://doi.org/10.1038/s41380-022-01498-7
Fleming, L. M., Jaynes, F. B., Thompson, S. L., Corlett, P. R., & Taylor, J. R. (2022). Targeted effects of ketamine on perceptual expectation during mediated learning in rats. Psychopharmacology, 239(8), 2395–2405. https://doi.org/10.1007/s00213-022-06128-2
DeMartini, K. S., Gueorguieva, R., Taylor, J. R., Krishnan-Sarin, S., Pearlson, G., Krystal, J. H., & O'Malley, S. S. (2022). Dynamic structural equation modeling of the relationship between alcohol habit and drinking variability. Drug and alcohol dependence, 233, 109202. https://doi.org/10.1016/j.drugalcdep.2021.109202
Groman, S. M., Thompson, S. L., Lee, D., & Taylor, J. R. (2022). Reinforcement learning detuned in addiction: integrative and translational approaches. Trends in neurosciences, 45(2), 96–105. https://doi.org/10.1016/j.tins.2021.11.007
Gianessi, C. A., Groman, S. M., & Taylor, J. R. (2022). The effects of fatty acid amide hydrolase inhibition and monoacylglycerol lipase inhibition on habit formation in mice. The European journal of neuroscience, 55(4), 922–938. https://doi.org/10.1111/ejn.15129
-
Thompson, S. L., Gianessi, C. A., O'Malley, S. S., Cavallo, D. A., Shi, J. M., Tetrault, J. M., DeMartini, K. S., Gueorguieva, R., Pittman, B., Krystal, J. H., Taylor, J. R., & Krishnan-Sarin, S. (2021). Saracatinib Fails to Reduce Alcohol-Seeking and Consumption in Mice and Human Participants. Frontiers in psychiatry, 12, 709559. https://doi.org/10.3389/fpsyt.2021.709559
Suthaharan, P., Reed, E. J., Leptourgos, P., Kenney, J. G., Uddenberg, S., Mathys, C. D., Litman, L., Robinson, J., Moss, A. J., Taylor, J. R., Groman, S. M., & Corlett, P. R. (2021). Paranoia and belief updating during the COVID-19 crisis. Nature human behaviour, 5(9), 1190–1202. https://doi.org/10.1038/s41562-021-01176-8
Groman, S. M., Lee, D., & Taylor, J. R. (2021). Unlocking the reinforcement-learning circuits of the orbitofrontal cortex. Behavioral neuroscience, 135(2), 120–128. https://doi.org/10.1037/bne0000414
Huang, G., Thompson, S. L., & Taylor, J. R. (2021). MPEP Lowers Binge Drinking in Male and Female C57BL/6 Mice: Relationship with mGlu5/Homer2/Erk2 Signaling. Alcoholism, clinical and experimental research, 45(4), 732–742. https://doi.org/10.1111/acer.14576
-
Groman, S. M., Ikemoto, S., Rushworth, M., Taylor, J. R., & Whelan, R. (2020). Introducing the PLOS ONE Collection on the neuroscience of reward and decision making. PloS one, 15(10), e0240505. https://doi.org/10.1371/journal.pone.0240505
Groman, S. M., Hillmer, A. T., Liu, H., Fowles, K., Holden, D., Morris, E. D., Lee, D., & Taylor, J. R. (2020). Dysregulation of Decision Making Related to Metabotropic Glutamate 5, but Not Midbrain D3, Receptor Availability Following Cocaine Self-administration in Rats. Biological psychiatry, 88(10), 777–787. https://doi.org/10.1016/j.biopsych.2020.06.020
Moin Afshar, N., Keip, A. J., Taylor, J. R., Lee, D., & Groman, S. M. (2020). Reinforcement Learning during Adolescence in Rats. The Journal of neuroscience : the official journal of the Society for Neuroscience, 40(30), 5857–5870. https://doi.org/10.1523/JNEUROSCI.0910-20.2020
Reed, E. J., Uddenberg, S., Suthaharan, P., Mathys, C. D., Taylor, J. R., Groman, S. M., & Corlett, P. R. (2020). Paranoia as a deficit in non-social belief updating. eLife, 9, e56345. https://doi.org/10.7554/eLife.56345
Bond, C. W., Trinko, R., Foscue, E., Furman, K., Groman, S. M., Taylor, J. R., & DiLeone, R. J. (2020). Medial Nucleus Accumbens Projections to the Ventral Tegmental Area Control Food Consumption. The Journal of neuroscience : the official journal of the Society for Neuroscience, 40(24), 4727–4738. https://doi.org/10.1523/JNEUROSCI.3054-18.2020
Groman, S. M., Hillmer, A. T., Liu, H., Fowles, K., Holden, D., Morris, E. D., Lee, D., & Taylor, J. R. (2020). Midbrain D3 Receptor Availability Predicts Escalation in Cocaine Self-administration. Biological psychiatry, 88(10), 767–776. https://doi.org/10.1016/j.biopsych.2020.02.017
Monsey, M. S., Ruiz, S. G., & Taylor, J. R. (2020). Regulation of Garcinol on Histone Acetylation in the Amygdala and on the Reconsolidation of a Cocaine-Associated Memory. Frontiers in behavioral neuroscience, 13, 281. https://doi.org/10.3389/fnbeh.2019.00281
Gianessi, C. A., Groman, S. M., Thompson, S. L., Jiang, M., van der Stelt, M., & Taylor, J. R. (2020). Endocannabinoid contributions to alcohol habits and motivation: Relevance to treatment. Addiction biology, 25(3), e12768. https://doi.org/10.1111/adb.12768
-
Groman, S. M., Keistler, C., Keip, A. J., Hammarlund, E., DiLeone, R. J., Pittenger, C., Lee, D., & Taylor, J. R. (2019). Orbitofrontal Circuits Control Multiple Reinforcement-Learning Processes. Neuron, 103(4), 734–746.e3. https://doi.org/10.1016/j.neuron.2019.05.042
Groman, S. M., Massi, B., Mathias, S. R., Lee, D., & Taylor, J. R. (2019). Model-Free and Model-Based Influences in Addiction-Related Behaviors. Biological psychiatry, 85(11), 936–945. https://doi.org/10.1016/j.biopsych.2018.12.017
Gianessi, C. A., Groman, S. M., & Taylor, J. R. (2019). Bi-directional modulation of food habit expression by the endocannabinoid system. The European journal of neuroscience, 49(12), 1610–1622. https://doi.org/10.1111/ejn.14330
Groman, S. M., Massi, B., Mathias, S. R., Curry, D. W., Lee, D., & Taylor, J. R. (2019). Neurochemical and Behavioral Dissections of Decision-Making in a Rodent Multistage Task. The Journal of neuroscience : the official journal of the Society for Neuroscience, 39(2), 295–306. https://doi.org/10.1523/JNEUROSCI.2219-18.2018
Torregrossa, M. M., MacDonald, M., Stone, K. L., Lam, T. T., Nairn, A. C., & Taylor, J. R. (2019). Phosphoproteomic analysis of cocaine memory extinction and reconsolidation in the nucleus accumbens. Psychopharmacology, 236(1), 531–543. https://doi.org/10.1007/s00213-018-5071-9